Our Products
HYPERKAT
Hydrogen Peroxide CatalystsHYPERKAT
Catalysts and Units for Decomposition of Hydrogen Peroxide Catalysts in Air Flows (Residual Content: < 0,1 ppm)
OZONKAT
Ozone CatalystsOZONKAT
Catalysts and Units for Decomposition of Ozone in Air Flows
(Residual Content: < 30 ppb)
CARBONKat
Carbon CatalystsCARBONKat
Catalysts and Units for
Cold Oxidation of Hydrocarbons
for Deodorization
HYPERKAT – Hydrogen Peroxide Catalysts
Catalytic Decomposition of Hydrogen Peroxide Catalysts (H2O2) in Air Flows
Chemical Decomposition Reaction of Hydrogen Peroxide
Hydrogen Peroxide strongly tends to decompose into water and oxygen, at that developing a great amount of heat according to the following formula:
2 H2O2 → 2 H2O + O2+ 196.2 KJ
However at room temperature and temperatures < 60 degree C, the rate of decomposition is immeasurably low so that hydrogen peroxide is practically permanent (metastable) in air. By using suitable catalysts, the decomposition process can be accelerated strongly so that complete decomposition of the hydrogen peroxide into the components water vapour and atmospheric oxygen can be reached in an air flow already at room temperature.
OZONKAT – Ozone Catalysts
Catalytic Decomposition of Ozone (O3) in Air Flows
Chemical Decomposition Reaction of Ozone
Ozone (Trioxygen O3) strongly tends to decompose into dioxygen (atmospheric oxygen O2), at that developing a great amount of heat.
2 O3 → 3 O2 + 285.6 KJ
The most characteristic property of ozone is its oxidizing power as a result of one of its 3 oxygen atoms.
O3 → O2 + O
Process of catalytic decomposition of ozone
At room temperature and temperatures < 60 degree C, the decomposition will proceed gradually. By using suitable catalysts, the decomposition process can be accelerated strongly so that complete decomposition of the ozone (O3) into atmospheric oxygen (O2) can be reached in an air flow already at room temperature.
The Catalyst
Our catalyst is a metal oxide mixture combined with an aluminosilicate in the form of pellets with the following characteristics:
- Surface of the pellets with adsorptive effect
- BET value: approx. 100 m²/g
- High dimensional accuracy
- High solidity of the pellets
The extremely high decay rate of the exhaust air to pass through is a result of the fineness of the pellets combined with the high adsorptive capacity of their surface.
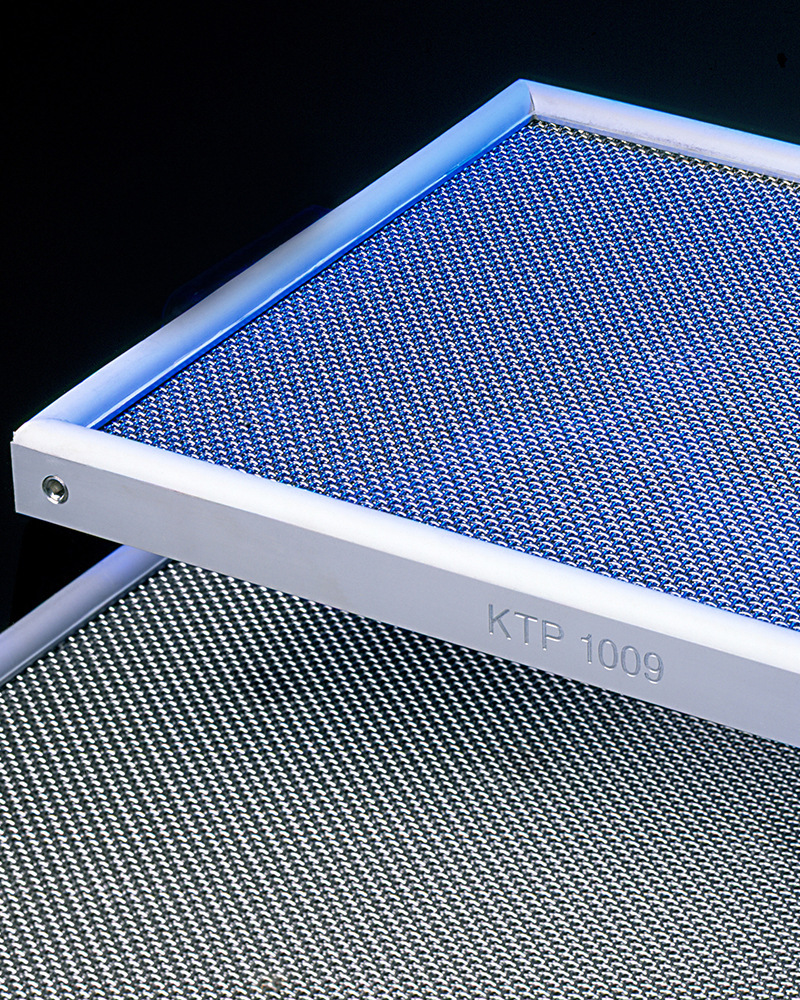
The Catalyst Panel
The catalysts are formed as a panel which is flown through by the hydrogen peroxide containing air flow.
The catalyst panel consists of aluminium profiles with embedded polished stainless steel wire cloth. The catalyst is available as pellets which are inserted into the catalyst frame. The catalyst panels are equipped on one or both sides with silicone rubber seals resistant to H2O2 .
Efficiency of the Catalyst Panel:
- At input concentrations of H2O2 up to 4000 ppm, the concentration of H2O2 in the clean air flow will fall to < 0,1 ppm.
- At input concentrations of O3 up to 1000 microgram, the concentration of O3 in the clean air flow will fall to < 30 microgram.
Configuration
The basic design of the system consists of a body with built-in pre-filter and catalyst panel. Fans have to be installed on suction side. Presently, we can offer you the following types of unit:
Small units up to 300 m3/h
Capacity: 1 catalyst panel KTP 300
Body: Consisting of ground stainless steel.
Large units up to 7200 m3/h
Capacity: 1-12 catalyst panel KTP 600
Body: A modified device of ventilation and air-conditioning meeting the quality standards of ventilation and air-conditioning.
Please contact us.
- Adress
- Hager Verfahrenstechnik GmbH
Gewerbestraße 7
66773 Schwalbach
Germany - info@hager-engineering.de
- Telephone
- +49 6834 – 780 40 80
- Fax
- +49 6834 – 780 40 81